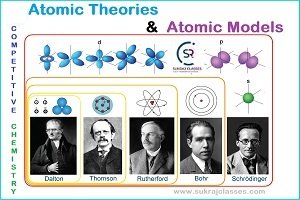
Atomic Theories and Atomic Models
Chemistry topic- “Atomic Theories and Atomic Models”, is important for all competitive exams like CET- Common eligibility Test, SSC CGL, SSC CHSL, RRB NTPC, UPSC and other state civil services exams. In these exams, almost 4-5 questions are coming from Chemistry. Let’s start topic:-
Atomic Theories and Atomic Models:-
During 18th and 19th centuries, many scientists attempted to explain the structure of the atom, but the main contribution in development of the modern atomic structure were by the scientists John Dalton, J.J. Thomson, Ernest Rutherford and Niels Bohr.
Dalton’s Atomic Theory:-
“The atomic theory” of matter was first proposed by John Dalton in 1808.
According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms.
The main point of this theory were:
- All matter is made of atoms.
- Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Note:- Dalton’s atomic theory was able to explain the law of conservation of mass, law of constant composition and law of multiple proportion very successfully.
Limitations of Dalton’s Atomic Theory:-
- The theory was unable to explain the existence of isotopes.
- Dalton atomic theory couldn’t explained the structure of atom.
J.J. Thomson’s Model of Atom:-
- J.J. Thomson model of atom based on cathode ray tubes experiment that was described in 1898.
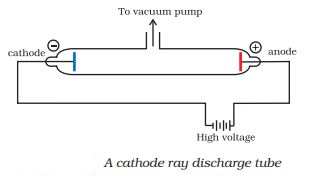
- Many different names are given to this model, for example:- plum pudding model or watermelon model.
Thomson proposed that: 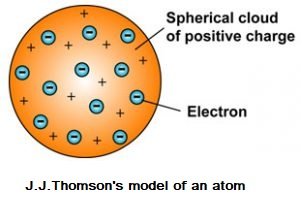
- An atom consists of a positively charged sphere and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Note:- J.J. Thomson awarded the Nobel Prize for the discovery of “electrons”.
Limitations of Thomson’s Atomic Structure:
- Thomson’s atomic model does not clearly explain the stability of an atom.
- Other subatomic particles, couldn’t be placed inside this atomic model.
RUTHERFORD’S MODEL OF ATOM:– 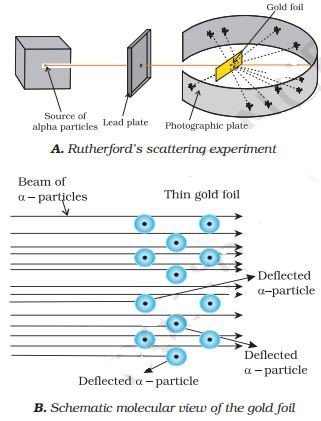
- His atomic model is based on the Alpha ray scattering experiment.
- He experimented with thin gold foil by passing alpha rays through it.
Main points of Rutherford nuclear model of an atom:-
- There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
- The electrons revolve around the nucleus in circular paths.
- The size of the nucleus is very small as compared to the size of the atom.
Note:- He discover another subatomic particle called “Nucleus”.
Drawbacks of Rutherford’s model of the atom:-
- The revolution of the electron in a circular orbit is not expected to be stable.
- The atom stay highly unstable.
BOHR’S MODEL OF ATOM:-
- Neils bohr brought up the model of atom in 1915.
- This is the most widely used atomic model to describe the atomic structure of an element.
- Neils bohr’s model is based on Planck’s theory of quantization.
The main point about this model of an atom:
- Electrons spin around the nucleus in an individualized separate path or unattached orbit.
- The electrons do not emit any energy while revolving in discrete orbits.
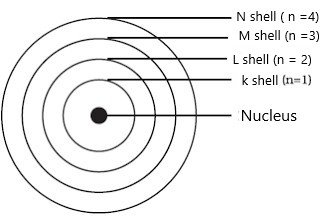
- These orbits or shells are called energy levels.
- These orbits or shells are represented by the letters K, L, M, N,… or the numbers, n=1,2,3,4,….
Limitations of Bohr’s Atomic Theory:
- Bohr’s atomic structure works only for single electron species such as H, He+ etc.
From these theories some new subatomic particles and definitions come out such as: electronic configuration, Orbit, Valence Electrons, valency, Proton, Neutron, electron, nucleus, Nucleons, atomic mass, atomic numbers, photon, Isotopes etc.
Read these topic at: Atomic structure.
Atomic Theories and Atomic Models
For More:
If you like and think that General Science (Chemistry) topic on “Atomic Theories and Atomic Models” was helpful for you, Please comment us. Your comments/suggestions would be greatly appreciated. Thank you to be here. Regards – Team SukRaj Classes.