States of Matter – Competitive Chemistry
“States of Matter – Competitive Chemistry” is important for all competitive exams like CET- Common eligibility Test, SSC CGL, SSC CHSL, RRB NTPC, UPSC and for other state civil service exams. In these exams, almost 4-5 Questions are coming from Chemistry. Lets start the Topic- States of Matter – Competitive Chemistry.
States of Matter – Competitive Chemistry
What is Matter?
Anything which has mass and occupies space is called matter. Everything around us are composed of matter for example – book, pen, pencil, water, air, all living beings etc.
- Matter basically exist in three physical states: – Solid, Liquid and Gas.
There are 2 more states of matter also exist, but these two states do not see in our everyday life. They are Plasma & Bose-Einstein condensate.
Different states of matter exhibit the following characteristics:
 (i) Solid: – Solids have definite volume and definite shape (In solids, these particles are held very close to each other).
(i) Solid: – Solids have definite volume and definite shape (In solids, these particles are held very close to each other).
(ii) Liquid: – Liquids have definite volume but not the definite shape. They take the shape of the container in which they are placed (In liquids, the particles are close to each other but they can move around).
(iii) Gases: – Gases have neither definite volume nor definite shape. They completely occupy the container in which they are placed (in gases, the particles are far apart and their movement is easy and fast).
Note:- These three states of matter are interconvertible by changing the conditions of temperature and pressure.
Classification of Matter:-
Matter can be classified as mixtures or pure substances.

Mixture:
Mixtures are made up of two or more substances that are not chemically combined with each other.
Examples:
- Sea water – a mixture of water and various salts.
- Air- a mixture of various gases like oxygen, carbon dioxide, nitrogen, argon, neon, etc.
Types of Mixtures:
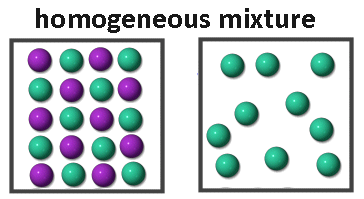
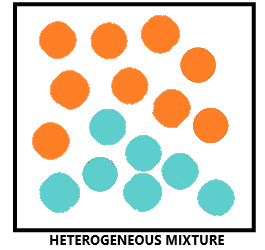
There are two main types of mixtures: homogeneous mixtures and heterogeneous mixtures.
- Homogeneous mixture: –In a homogeneous mixture, the components completely mix with each other and its composition is uniform throughout. For example: Sugar solution, and air.
- Heterogeneous mixtures: – In heterogeneous mixtures, the composition is not uniform throughout and sometimes the different components can be observed. For example: mixtures of salt and sugar, grains and pulses along with some dirt (often stone) pieces.
Pure Substances:
- Pure substances have characteristics different from the mixtures.
- Pure substances have fixed composition.
- Examples: Copper, silver, gold, water, glucose etc.
- Glucose contains carbon, hydrogen and oxygen in a fixed ratio and thus, like all other pure substances has a fixed composition.
Pure substances can be further classified into Elements and Compounds. 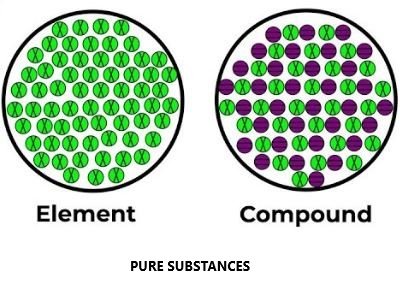
Elements:-
- An element consists of only one type of particles. These particles may be atoms or molecules.
- Examples: – Sodium, copper, silver, hydrogen, oxygen etc.
- They all contain atoms of one type.
Note: – However, the atoms of different elements are different in nature. Some elements such as sodium or copper, contain single atoms held together as their constituent particles whereas in some others, two or more atoms combine to give molecules of the element.
- Thus, hydrogen, nitrogen and oxygen gases consist of molecules in which two atoms combine to give their respective molecules.
Compound:-
When two or more atoms of different elements combine, the molecule of a compound is obtained. For examples: – water, ammonia, carbon dioxide, sugar etc.
Dry Ice:
Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
- Thus, we can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
Diffusion:
The mixing of a substance with another substance due to the motion or movement of its particles is called diffusion.
- The diffusion of one substance into another substance goes on until a uniform mixture is formed. Diffusion takes place in gases, liquids and solids.
Latent Heat:
Latent heat is the heat energy which has to be supplied to change the state of a substance. Latent heat does not increase the temperature of a substance.
Sublimation:
Sublimation is the change of gaseous state directly to solid state without going through liquid state, and vice versa. For Example:- Ammonium Chloride, Camphor & Iodine.
- When these solid substances are heated, their particles move quickly and they separate completely to form vapour (or gas). Similarly, when these vapour (or gas) is cooled, these particles slow down so quickly that they become fixed and form a solid.
Evaporation:
Evaporation is the process of conversion of a liquid into vapour (or gas) at its boiling point.
- Temperature, surface area of liquid, humidity and wind speed are factors that affect evaporation. Evaporation causes cooling effect.
Fusion: Heat energy required to change 1kg of solid into liquid.
Vaporization:
Vaporization is a process of conversion of a substance from the liquid or solid phase into the gaseous (vapour) phase.
- If conditions allow the formation of vapour bubbles within a liquid, the vaporization process is called boiling.
- Heat energy required to change 1kg of liquid to gas at atmospheric pressure at its boiling point.
For More:
States of Matter – Competitive Chemistry
If you like and think that General Science (Chemistry) topic on “States of Matter” was helpful for you, Please comment us. Your comments/suggestions would be greatly appreciated. Thank you to be here. Regards – Team SukRaj Classes.