Heat (ऊष्मा) and Temperature (तापमान)
Physics topic-“Heat (ऊष्मा) and Temperature (तापमान)”, is important for all competitive exams like CET- Common eligibility Test, SSC CGL, SSC CHSL, RRB NTPC, UPSC and for other state civil services exams. In these exams, almost 4-5 questions are coming from Physics. Let’s start the topic- Heat (ऊष्मा) and Temperature (तापमान).
Heat (उष्मा):
Heat can be defined as a form of energy that can be transferred from one body to the other or within the body itself with a temperature difference and can be generated by a body at the expense of other forms of energy.
Heat energy is the result of the movement of tiny particles called Atoms, molecules or ions in solids, liquids and gases. Heat is the transfer of energy from one object to another. The transfer or flow due to the difference in temperature between the two objects is called Heat.
Transferring of Heat When two items are combined or touching each other, their molecules will transfer energy called heat. They will try to come to a point where they both have the same temperature. This is called Thermal Equilibrium.
For example: – when we take an ice cube and put it into a glass of warm soda. If we put the ice in the soda, the soda (which is warmer) will transfer some of its heat energy to the ice. The ice cube will become warmer and melt, while the soda will cool down. At this stage soda and water from the ice will be at the same temperature, this is called the stage of thermal equilibrium.
Some important points:-
- The SI unit of heat energy is Joule abbreviated as ‘J’.
- Where 1 Joule = 1 Newton × Meter.
- Heat is measured by the Calorimeter.
- Heat is represented by “Q”.
- When heat absorbed by surrounding, a man feels cold.
- When heat get from surrounding, a man feels hot.
Mode of Heat transfer: –

Conduction (ताप चालन): 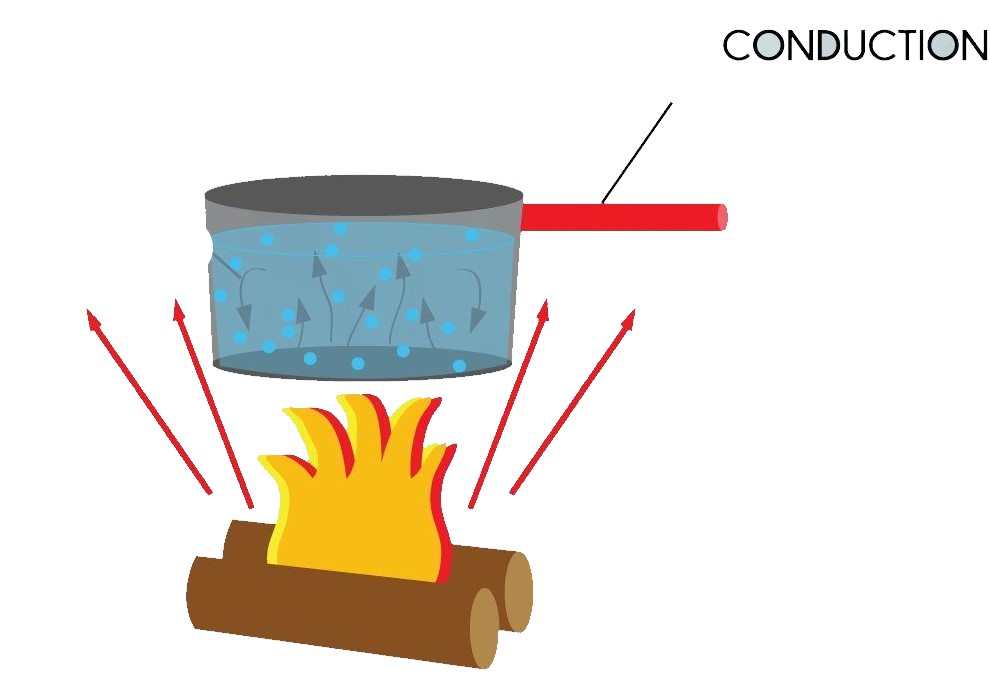
In this mode heat transfer when two objects come in direct contact or heat takes place between atoms and molecules in direct contact.
Eg: cooking of food etc.
Convection (संवहन):
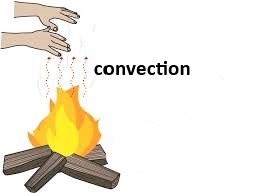 In Convection, process by which heat is transferred by a medium such as air or water.
In Convection, process by which heat is transferred by a medium such as air or water.
Radiation (विकिरण):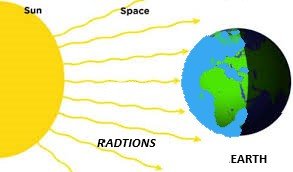
It is the method in which the transfer of heat takes place without any medium or in vacuum.
Eg: heat energy comes from sun to earth.
Important FACTS of Heat :
1) Black and rough surface feels more heat.
2) Two blankets are warmer than single one because air present between them act as insulator of heat.
3) When a metabolic sphere heated, their area will increase.
4) When hole in a metallic sheet, make heated, its size increase.
5) If a door of freeze open in a room, the temperature of room gradually increase.
6) Freezer always feted in upper part of freeze, to keep away compressor heat.
7) After accumulation of ice in freezer, freeze does not cool properly, because ice is bad conductor of heat. Hence, advice to defrost freeze.
8) In freeze gas used – Ammonia Gas.
9) All automatic electrical heating devices, auto cut by using the “Thermostat”.
10) A.C. and freeze cools by the “vaporization” of volatile liquid.
11) A.C. controlled temperature as well as relative humidity of the room.
12) Cooler, pitcher cools by the process of “evaporation” of water. It does not cool in rainy season because atmospheric humidity too much, the evaporation of water does not occur at the rainy season.
13) When water heated above Zero Degree, the density firstly increase then decrease.
14) When water heated above Zero Degree, the volume firstly decrease then increase.
15) When Ice melts, The Volume of the mixture of ice and water does not change.
Latent Heat (गुप्त ऊष्मा):-
Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature.
- The latent heat is that amount of heat (in units of joules or calories) per mole or unit mass of the substance to change one state into other state.
![]()
Important FACTS related to Latent Heat: –
1) All the ice does not melt at-once at hills tops (glaciers), due to high latent heat.
2) Water vapours serve more burn than boiled water due to high latent heat.
3) Water has a fix Boiling point at 100 degree, due to latent heat.
4) Freezing point of water is fix at Zero degree, due to latent heat.
Specific Heat (विशिष्ट ताप):-
Specific heat is the ratio of the quantity of heat required to raise the temperature of a body by one degree to that required to raise the temperature of an equal mass of water by one degree.
Important FACTS about Specific Heat: –
1) Water is use in boilers, due to it’s high specific heat.
2) Water is also use in rubber bladder for sacking purpose due to it’s high specific heat.
Some Important Definitions:
Boiling point (क्वथनांक बिंदु): Boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure of the liquid environment.
- At this temperature, the liquid is converted into a vapour.
- The boiling point of the liquid depends upon the pressure of the surrounding. When the liquid is at high pressure, it has a higher boiling point than the boiling point at normal atmospheric pressure.
- Water has a fix Boiling point at 100 degree, due to latent heat.
Melting point (गलनांक बिंदु): The melting point is usually defined as the point of temperature at which materials changes from solid to liquid.
Freezing point (हिमांक बिंदु): It is the temperature at which a liquid becomes a solid.
- Freezing point of water is fix at Zero degree, due to latent heat.
Triple point (त्रिक बिन्दु) of water:
Triple point of water is defined as the temperature and pressure at which liquid water, solid ice and water vapour can coexist in a stable equilibrium.
- At triple point of water, both solid (ice) and vapour state are present, so we can say that boiling point and freezing point become same.
- 4 temperature, is also known as triple point of water because on this temperature water can stable in all three state – Ice, Water, Gas.
- At 4 temperature water have maximum density.
Condensation (संघनन): It is the process of change of physical state of a matter from the gas phase into the liquid phase
- It is the reverse of vaporization.
For More⇓:
Heat (ऊष्मा) and Temperature (तापमान)
TEMPERATURE (तापमान): –
Temperature is the degree of hotness or coldness of a body or environment.
A measure of the warmth or coldness of an object or substance with reference to some standard value.
Temperature can be defined also as temperature is a measure of the average kinetic energy of the particles in an object. When temperature increases, the motion of these particles also increases.
Important FACTS about Temperature:
1) Its SI unit is “Kelvin”.
2) It is measured by Thermometer.
3) It is represented by “T”.
4) Thermometers are calibrated in various temperature scale. The most common scales are the Kelvin, Fahrenheit, and Celsius scale.
Thermometer (तापमापी): –
Temperature is measured by the thermometer.
1) Clinical or Medical thermometers: 
- A Medical thermometer is used to measure body temperature.
- These kinds of thermometers are used in the clinics by the doctors, so they are also called a doctor’s thermometers.
- It is used to measure human body temperature in the range of 35 °C to 42 °C
- In this thermometer we use mercury.
Why mercury used in thermometer:
1) Greater visibility.
2) Higher density.
3) It not stick with the wall of container glass.
4) Uniform expansion on heating.
Limitation of clinical thermometer: It cannot measure high temperature.
2) Resistance Thermometer:- 
- It is used to measure the high temperature.
- It is used in industries.
- Platinum is use in this thermometer as resistance.
- Platinum is known as “Adam – catalyst”.
3) Gas Thermometer: –

- It is use to check accuracy of the correct reading of the thermometers.
- It is use in scientific purpose.
- It is most accurate thermometer.
4) Alcohol temperature: 
- Use for measuring lower – temperature.
- “Ethyl alcohol” use in it, because it has highest freezing point.
- Study of “lower temperature” is called “Cryology”.
Cryogenic Engine :
In rocket cryogenic engine use.
Jet engine cannot fly in the space because jet engine takes oxygen from atmosphere. While rocketing engine can enter in the space because rocket have their own oxygen cylinder and enters in the space.
Pressure (दाब): –
Pressure is the force applied perpendicular to the surface of an object per unit area.
- The SI unit of pressure is = Pascal (Pa).
Important FACTS about Pressure:
1) With increase in pressure, the boiling point of water increase. While, decrease in pressure boiling point of water also decrease.
2) Pressure cooker cooked the food earlier, due to high pressure.
- The pressure inside the pressure cooker is 2- Atmo
- Temperature of water/ Boiling point of water inside pressure cooker, goes more than 110º .
- Cooking of food on hills, takes more time than usual due to low pressure on height.
3) By adding salt in water, it’s boiling point get increased.
4) Increment in Pressure also increase the Boiling point.
5) By adding salt or by increasing pressure on ice, their melting point increase. That’s why kulfi – maker, mix salt in ice.
Regelation (पुनर्हिमायन ):-
When two pieces of ice – cube pressed together, they joint in single one due to process of regelation. 
- Ice always melt from the top of the hills because there is low atmospheric pressure due to which low regelation process takes place.
- Skating on ice is possible due to process of regelation.
Heat (ऊष्मा) and Temperature (तापमान)
If you like and think that General Science (Physics) topic- “Heat (ऊष्मा) and Temperature (तापमान)” was helpful for you, Please comment us. Your comments/suggestions would be greatly appreciated. Thank you to be here. Regards – Team SukRaj Classes.